
Top Performing Drug – Enbrel (June Edition)
Shots:
- In continuation of our previous series on the top-performing drug of the month, based on 2021 revenue, this month we have covered Enbrel as the top-performing drug of the month
- Enbrel is a biologic drug classified as a TNF inhibitor used to treat rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and other autoimmune diseases
- PharmaShots presents a detailed take on the key features of Enbrel with a detailed analysis of its revenue, clinical trials, alternatives, and approvals. The report is concluded with an engaging SWOT analysis and informative KOL reviews
Active Ingredient: etanercept
Dosage Forms & Strengths:
- 50 mg Single-use Prefilled Syringe
- 50 mg Single-use Prefilled SureClick Autoinjector
- 25 mg Single-use Prefilled Syringe
- 25 mg Multiple-use Vial
Mechanism of Action: Tumor Necrosis Factor (TNF) blocker
Originators: Immunnex
Licensors: Amgen and Pfizer
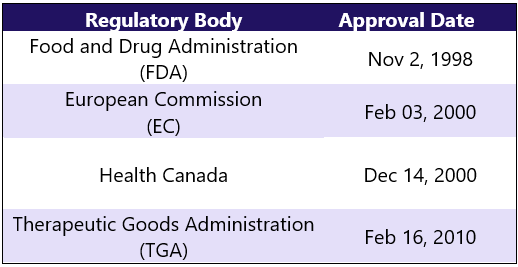
First Approvals: Below are the first approvals of Enbrel from different regulatory agencies.
Revenue Analysis1
PharmaShots presents a 5-year analysis of Enbrel sales. Before we deep dive into its sales analysis of each year, let us first look at how this product was launched and its related collaborations.
Amgen and Pfizer collaboration
Enbrel was launched in November 1998 by Immunex. Before this, Immunex entered an agreement with Wyeth to promote Enbrel in the U.S. and Canada. In July 2002, Amgen acquired the rights to Enbrel as part of its acquisition of Immunex. In connection with this, Amgen agreed with Wyeth to amend and restate an existing long-term Enbrel promotion agreement between Wyeth and Immunex. Under this agreement, Wyeth and Amgen jointly marketed and sold Enbrel to all appropriate segments in the U.S. and Canada for all approved indications other than oncology. The rights to promote Enbrel in the U.S. and Canada for oncology indications were reserved for Amgen. In 2009, Pfizer acquired Wyeth thus seeking the rights to Enbrel. The co-promotion collaboration agreement between the two companies in the United States and Canada expired on October 31, 2013. Amgen now has full ownership of Enbrel promotional rights in the United States and Canada while the rights to market Enbrel outside the United States and Canada are reserved for Pfizer.
Although, the sales of Enbrel have been decreasing continuously over the past 5 years, it has been holding its spot in the top-performing drugs.
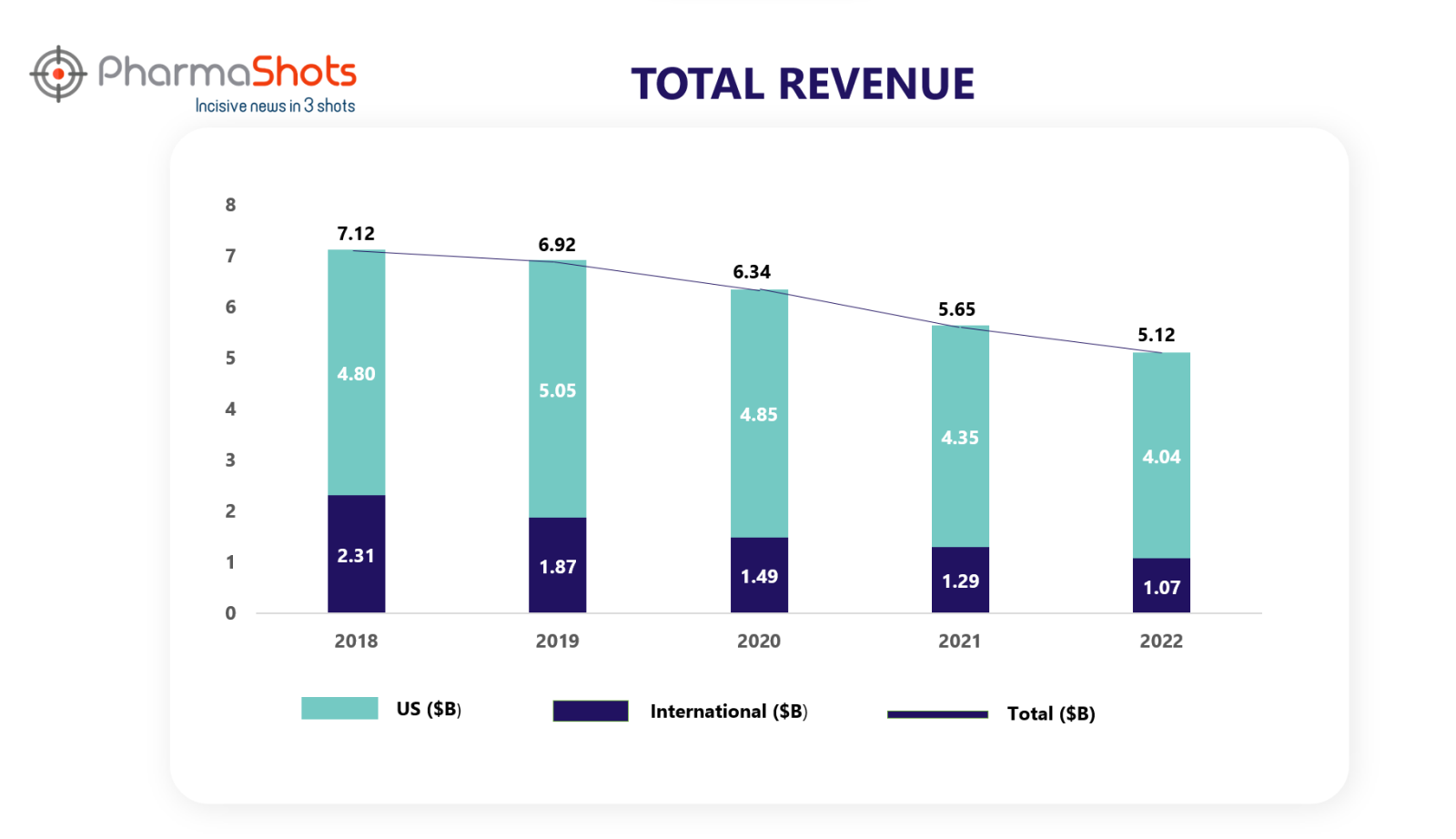
The decrease in Enbrel sales for 2022 was primarily driven by unfavorable changes to estimated sales deductions, lower volume, and lower net selling prices.
In 2020 and 2021, this decrease was driven by lower net selling price, unit demand, and unfavorable changes in inventory, partially offset by favorable changes to estimated sales deductions and inventory. Consistent with prior periods, Enbrel continued to lose market share, and this decline was compounded by a reduction in the growth rate of the rheumatology market as a result of COVID-19. The increase in ENBREL sales for 2019 vs 2018 was primarily driven by favorable changes to estimated sales deductions and an increase in net selling price, partially offset by lower unit demand.
*International sales included sales from Canada and other countries.
Approved Indications for Enbrel2
Enbrel is a tumor necrosis factor (TNF) blocker indicated for the treatment of:
- Rheumatoid Arthritis (Moderate to Severe): The drug is used for reducing signs and symptoms, preventing deep joint damage, and improving physical functions.
- Polyarticular Juvenile Idiopathic Arthritis (Moderate to Severe): The drug reduced signs and symptoms in patients aged 2 years or older
- Psoriatic Arthritis: The drug reduces signs and symptoms, keeping joint damage from getting worse, and improving physical function in patients
- Ankylosing Spondylitis: The drug reduces signs and symptoms in patients with active ankylosing spondylitis.
- Plaque Psoriasis (Moderate to Severe): The drug is used for children aged 4≥yrs and adults
Clinical Trials Analysis3
Currently, Enbrel is undergoing 541 trials, incl. 334 industry trials, 238 of which are interventional and 96 observational trials.
PharmaShots' analysis of Enbrel’s industry trials is depicted below: (Trials were taken on 6 Jun 2023).
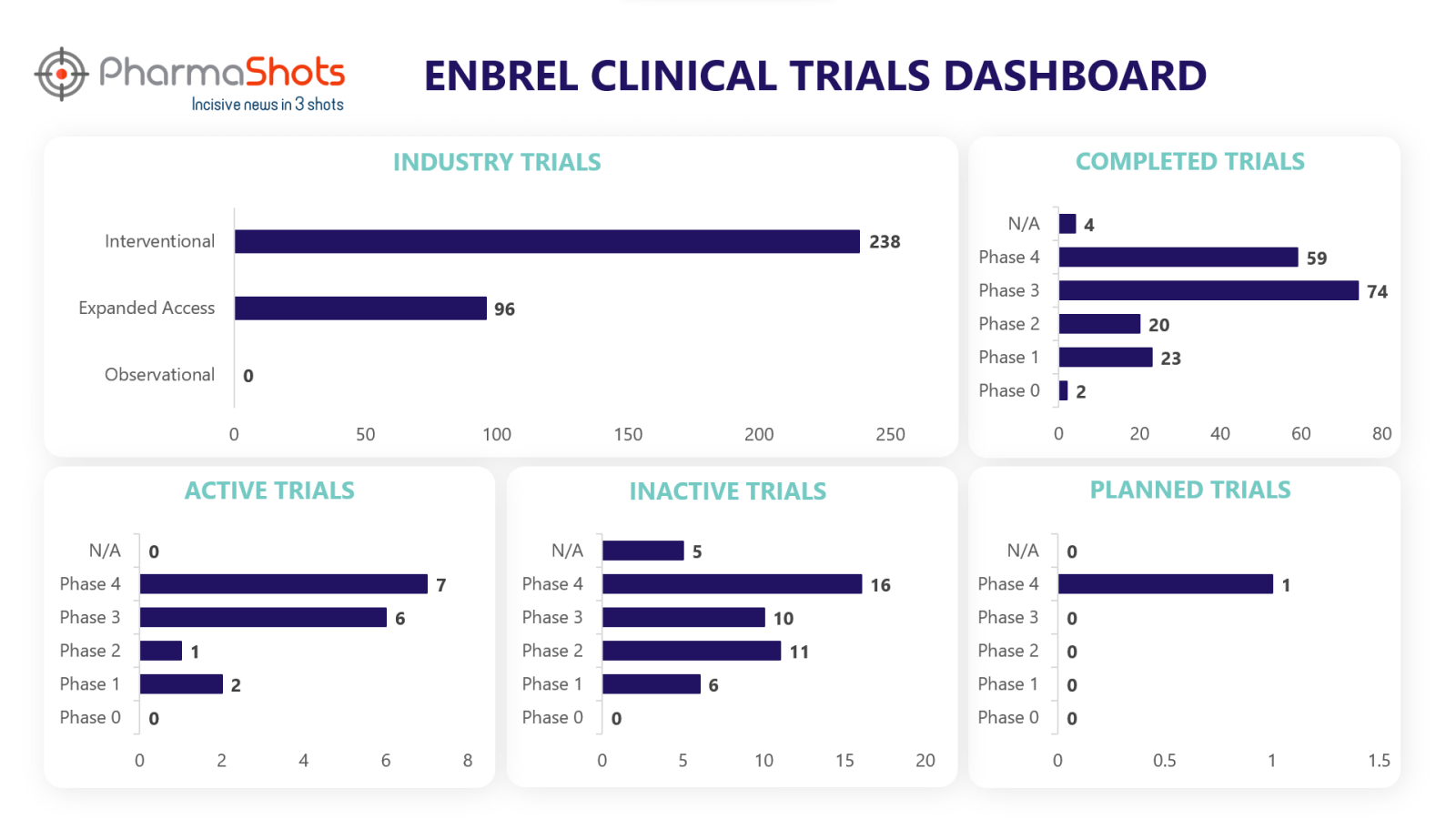
*Active trials include Recruiting; Active, Not Recruiting; Enrolling by Invitation, and suspended
*Inactive trials include Terminated; Withdrawn; Unknown Status
*Planned trials include Not, yet recruiting
Enbrel Trials Representation (Country-wise)4
Presently, Enbrel is being evaluated under different indications across the globe. The stacked column chart below is a visual representation of the clinical trials conducted with Enbrel so far. We have showcased industrial trials (only interventional studies) of the top 10 countries worldwide. (The chart depicts data till 8th Jun 2023).
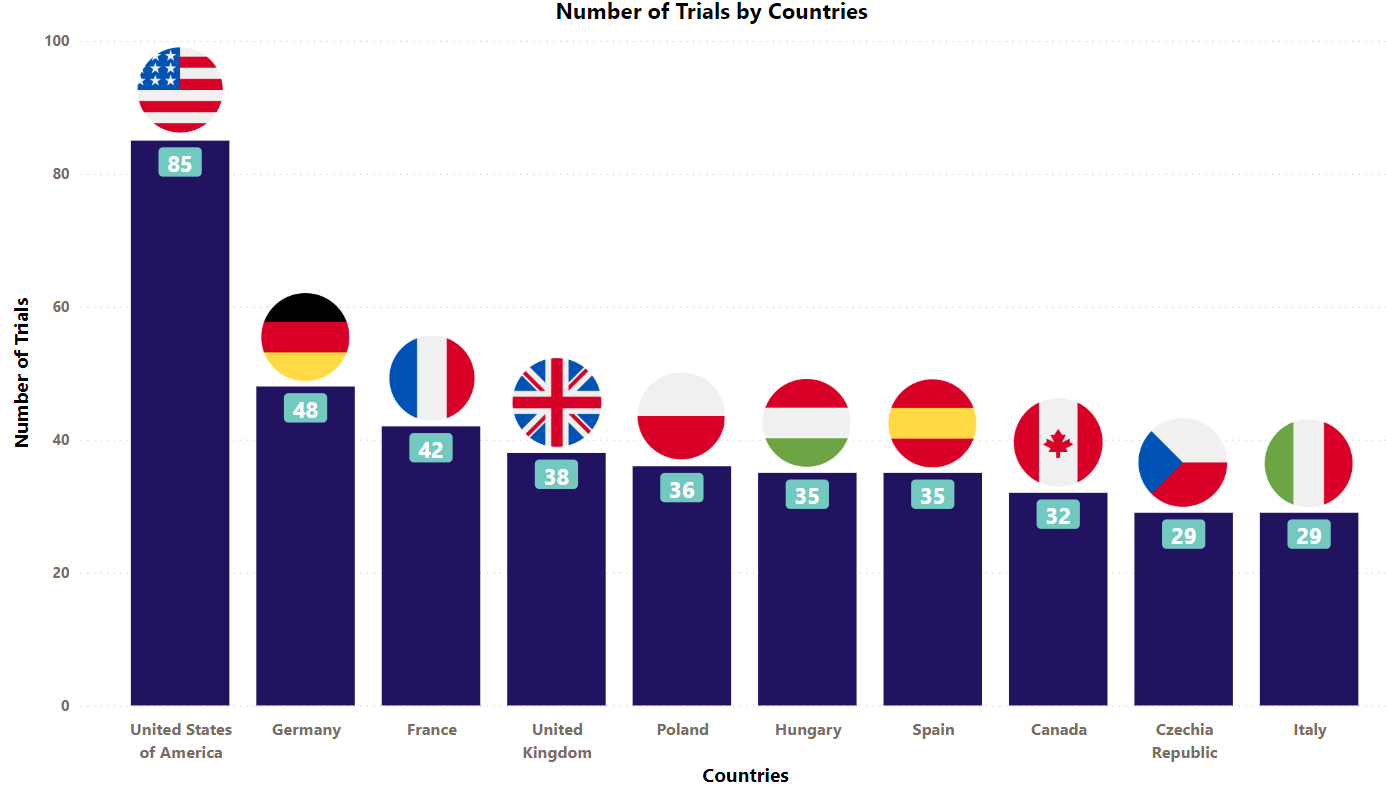
* Besides the data shown in the chart, 29 countries have double-digit trial numbers, and 38 countries with single-digit trial numbers. For a detailed report on it, mail us at connect@pharmashots.com
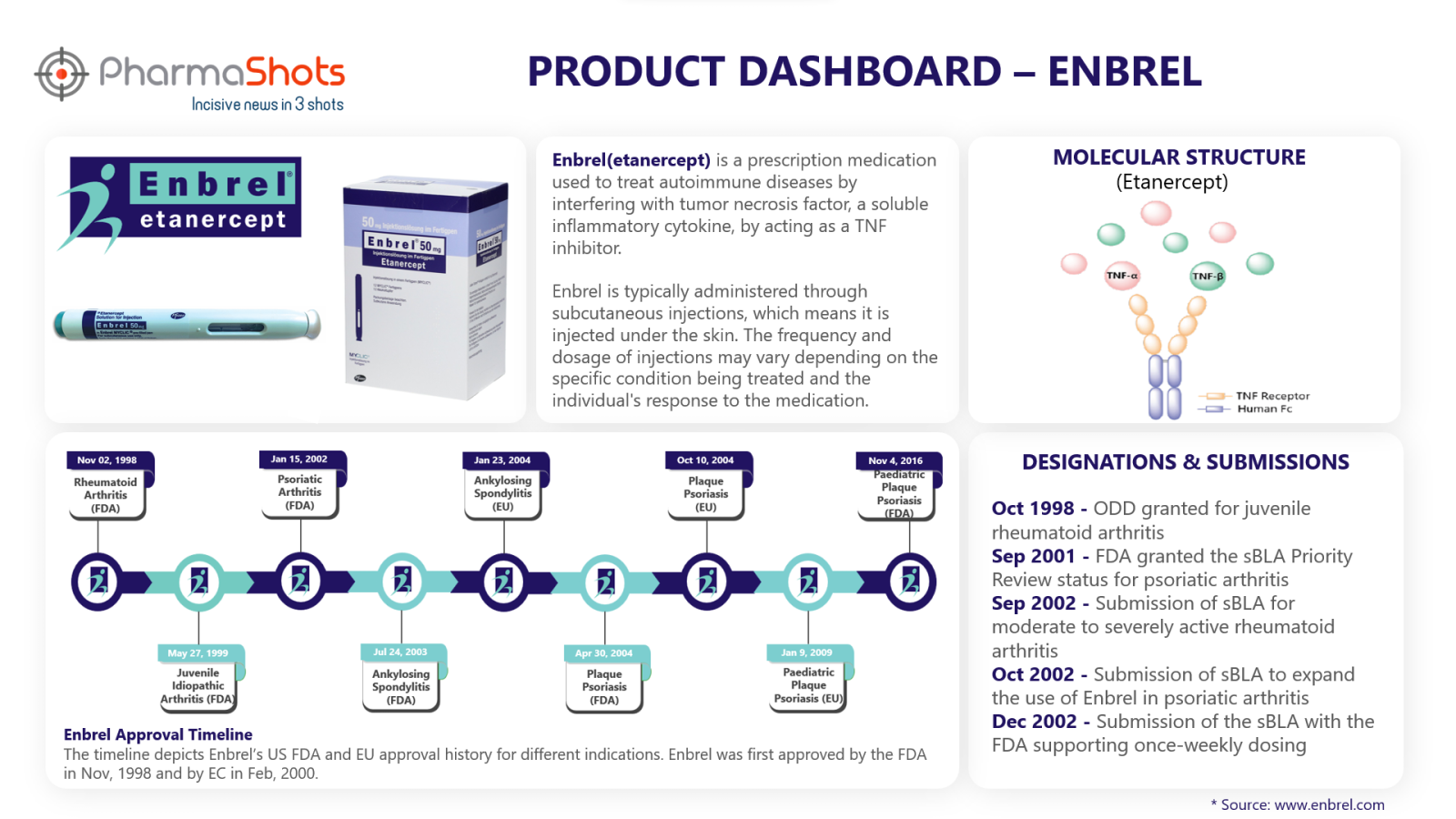
Product Dashboard
Enbrel key metrics and data through its product dashboard prepared by PharmaShots.
Enbrel Alternative Drugs5
In response to Enbrel, several other drugs are available in the market and are used to treat different indications. Some of the alternative drugs for Enbrel include:
.png)
Enbrel’s Biosimilars6
As Enbrel has a long journey in the market it is already facing competition from biosimilars in the US and other countries. Some of its approved biosimilars are mentioned below along with the regulatory bodies they are approved by*.
.png)
* This list is not an exhaustive list. If you are looking for an exhaustive list of Enbrel biosimilars, feel free to reach us at connect@pharmashots.com with the subject line “Enbrel biosimilars”
Enbrel’s SWOT Analysis7
.png)
Strengths:
Efficacy: Enbrel is highly effective in treating autoimmune diseases such as rheumatoid arthritis, psoriasis, and psoriatic arthritis. It can provide significant relief from symptoms, reduce inflammation, and improve the overall quality of life for patients.
Long Track Record: Enbrel has been on the market for several years. Thus, it has clinical research and data to support its effectiveness and safety
Multiple Indications: Enbrel is approved for the treatment of several autoimmune conditions, giving it a broader market reach and potential for revenue generation.
Biologic Advantage: As a biologic drug, Enbrel offers a targeted approach by inhibiting specific proteins involved in the immune response, etanercept can modulate biological responses that are induced or regulated by TNF, including expression of adhesion molecules responsible for leukocyte migration that leads to a more precise treatment compared to traditional therapies
Convenient Drug: Enbrel is available as an autoinjector, and administration is easy to learn making it a handy therapy option. Also, The Enbrel Mini cartridge with AutoTouch autoinjector is a reusable autoinjector designed with patients in mind, with a comfortable gray finger grip, an easy-to-press status button, and a needle designed to remain hidden throughout the injection process
Weaknesses:
High Cost: Enbrel is an expensive medication, which can limit access for patients who do not have adequate insurance coverage or financial resources
Administration: Enbrel is typically administered through subcutaneous injections, which makes it inconvenient for some patients
Competition from other drugs: Enbrel faces significant competition from other biologic drugs and newer therapies in the autoimmune disease market. This can impact its market share and revenue potential. Its main competitor drugs include Remicade, Humira, Stelara, Otezla
Side Effects: Enbrel can cause mild or serious side effects respiratory infections, injection site reactions, memory loss, etc.
Opportunities:
Emerging Markets: The growing demand for effective treatments for autoimmune can bring an opportunity for Enbrel to expand its market presence globally
New Indications: Exploring and obtaining approvals for additional indications can help Enbrel to increase its market potential
Combination Therapies: Enbrel is combined with other therapies for the treatment of rheumatoid arthritis, ankylosing spondylitis, or psoriatic arthritis which can help in making symptoms less severe
Expanded Formulation: Enbrel is administered by subcutaneous injection. Its expansion in different formulations can provide ease for the patients in the administration of the drug
Threats:
Patent Expiration: Enbrel's patent protection expired in 2012 which opened the entry for generic and biosimilar competitors to enter the market, potentially eroding its market share and revenue
Regulatory Challenges: Changes in regulatory requirements or safety concerns could impact Enbrel's ability to maintain its market position
Development of new therapies: Advancements in research and the introduction of new therapies for autoimmune disease treatments can affect Enbrel’s market share
Patient Stories8
Patient stories are powerful narratives that provide invaluable insight into the human experience of illness, recovery, and healthcare journeys. Each patient's story is unique, offering a glimpse into the challenges and emotions associated with navigating the complex world of healthcare. Some of the patients’ stories for Enbrel are mentioned below:
- SARAH’s STORY: Until two years ago Sarah had very bad arthritis and was in severe pain. Then, she took the anti-TNF treatment' Enbrel. Sarah describes Enbrel as 'completely and totally changing her life'. She said that her quality of life has improved and although things are not 'brilliant' she has far more mobility than before starting on Enbrel
- LISA’s STORY: Lisa shared her 50-year perspective of living with ankylosing spondylitis. The symptoms started when she was 13. When other treatments didn’t work, Lisa started to take Enbrel. She says “For me, relief from my symptoms was immediate, and I kick myself to this day for being so resistant to giving myself a shot, which turned out to not be a big deal after all”
- BRENDA’s STORY: Brenda was diagnosed with Rheumatoid Arthritis in 1991. Within a year, her ankles were so swollen it became hard to get into shoes. In 2000, she started to take Enbrel. Kleinsasser says, “I really do believe this is what’s helped me keep working full-time”
KOL* Reviews9
KOL reviews provide insights into various products and services. Generally, these reviews are helpful when consumers research the product and read multiple reviews before buying it. Below are some of the KOL reviews for Enbrel.
- In Jul 2003, when Enbrel was improved for Ankylosing spondylitis, Kevin Young, VP of Amgen's Inflammation Business Unit said "The approval of ENBREL for the treatment of AS is truly exciting, offering many patients significant relief of symptoms such as back pain, morning stiffness and fatigue as rapidly as two weeks after initiation of therapy. Also, for the first time, we see improvement in spinal mobility which is a debilitating symptom of the disease."
- Sean E. Harper, EVP of R&D at Amgen said "The need for an effective treatment for chronic moderate-to-severe pediatric psoriasis patients is high, and safety is always a concern when it comes to treating children. ENBREL has over a decade of experience in adult moderate to severe plaque psoriasis, and that proven track record matters to healthcare professionals, as well as the parents of children with moderate-to-severe plaque psoriasis. Today's FDA approval shows that innovation doesn't stop with a drug's first market approval, and further reflects Amgen's commitment to continually unlock and expand the therapeutic potential of our medicines in the hopes of filling unmet patient needs."
- Yvonne Greenstreet, SVP and Head of the Medicines Development Group for Pfizer said, “With its first approval for RA in 1998, ENBREL has 2.5 million patient-years of collective clinical experience, and we continue to gain important knowledge about these conditions and the potential benefits of treating patients with certain chronic inflammatory diseases.”
- Dr. Victoria Kusiak, North American medical director of Wyeth Pharmaceuticals said, "We are pleased to now be able to offer ENBREL to physicians and patients battling ankylosing spondylitis. ENBREL has a history of proven efficacy and tolerability which will be important to physicians introducing the therapy to a new group of patients."
- Josef S. Smolen, Chairman of the Department of Rheumatology, Medical University of Vienna, Austria said, “The medical community has recently questioned whether clinical remission or LDA could be sustained if a biologic agent is discontinued. These data show that patients with moderately active disease who achieve LDA or clinical remission with ENBREL treatment may lose these clinical benefits if ENBREL is discontinued.”
* Key Opinion Leaders (KOLs) are crucial when it comes to the launch and assessment of pharmaceutical products. At Octavus, we recognize the importance of KOLs in the industry, which is why our proficient team dedicatedly tracks their activities and provides valuable insights to the pharma fraternity.
We understand that KOL tracking and selection can be overwhelming and time-consuming. That's why we offer extensive KOL tracking services to help our clients stay ahead of the curve. Our team of experts can provide you with the latest information on KOL activities, including their opinions, publications, and affiliations.
Interested in learning more about our KOL tracking services? Don't hesitate to reach out to us at bd@octavusconsulting.com or connect@pharmashots.com. We would be more than happy to provide you with more information and discuss how our services may benefit your business.
Octavus is a dedicated consulting company that offers a one-stop market solution to life science enterprises, biopharma, MedTech, diagnostic centers, digital health companies, animal healthcare, and start-ups.
References:
1. Sec fillings
2. Enbrel Prescribing Information
4. Enbrel Trials Representation (Country-wise)
5. Enbrel Alternative drugs
6. Enbrel biosimilars
7. Enbrel’s SWOT Analysis
8. Patient stories
9. KOL reviews
Related Post: Top Performing Drug – Trikafta/Kaftrio (May Edition)
Tags

Senior Editor at PharmaShots. She is curious and very passionate about recent updates and developments in the life sciences industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots.














